Abstract
Introduction: Thrombopoietin receptor agonist eltrombopag (ELT) provides hematologic improvement in up to 50% of adults with acquired aplastic anemia (AAA) refractory to immunosuppressive therapy (IST). However, current data for ELT efficacy and tolerance in childhood AAA is very limited.
Methods: We conducted a multicenter nationwide retrospective study on behalf of the French Reference Center for Aplastic Anemia. Patients under 18 treated with ELT after IST for AAA were included. Lineage responses were defined as follows: red cells transfusion independency or hemoglobin increase of 1.5 g/dL, platelets transfusion independency or a 20 G/L increase in platelets counts, doubling of the neutrophil count or an increase above 0.5 G / L.
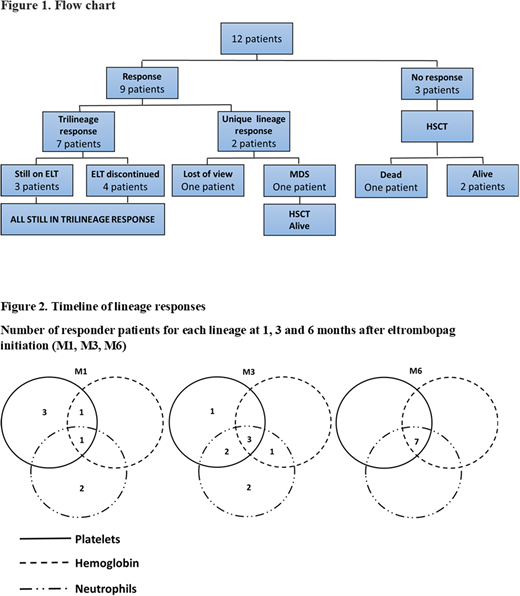
Results: We identified 12 children (2-17 years old, mean 9.5±5.9 years) treated for severe (7) /very severe (5) AAA with horse ATG and cyclosporine as first-line treatment between 2014 and 2018 in five different centers. Minor PNH clone was identified at diagnosis in two patients. All patients had a normal bone-marrow karyotype and FISH. None had a matched related donor. Median time between IST onset and eltrombopag initiation was 3.5 months [1-46]. All patients still met severe aplastic anemia criteria and needed RBC and platelet transfusions at ELT initiation. Mean hemoglobin, reticulocytes, platelets and neutrophils values were 7.3±0.8 g/dL, 24.5±24 G/L, 11±5.9 G/L and 0.8±0.7 G/L. Seven patients had received IST for more than 3 months and were therefore considered refractory to this treatment. ELT was indicated for an uncontrolled bacterial infection in 2 patients one and two months after IST initiation. One patient received an excessive dose of 12.5 mg/kg/day. Average ELT dosage for the other patients was 2±0.6 mg/kg/d. The median duration of treatment with ELT was 5.5 months [1-10]. After ELT initiation, nine patients (75%) reached hematological response for at least one cell line with no additional treatment. In these patients, hemoglobin increased on average by 3±1.3 g/dL, neutrophils by 2.5±3.5 G/L and platelets by 89.8±39.7 G/L. Seven patients (58%) achieved a trilineage response and were both RBC and platelets transfusions independent after 1 month (1), 3 months (3) and six months (7). Five patients (41.6%) reached a robust response (platelets > 50 G/L, hemoglobin > 10 g/dL, neutrophils > 1 G/L). All these trilineage responders were still on cyclosporine at the time of last assessment. ELT was withdrawn in 4 trilineage responders. Two of them still fulfilled robust response criteria 3 and 43 months after ELT withdrawal while the 2 others required occasional transfusions. The 3 others trilineage responders who were still receiving ELT remained transfusion independent 6 (2) and 11 (1) months after ELT initiation. Two patients (16.6%) had a unique lineage response: one was lost of view and one developed a myelodysplasia with RUNX1 mutation 3 months after ELT initiation. He is alive 8 months after HSCT. No clonal evolution was reported in the 5 other patients for whom cytogenetics follow-up was available. No response was achieved in 3 patients (25%), all of whom were treated for less than 3 months: ELT was withdrawn for deemed inefficiency in 2 patients (16.6%) after 1 and 2 months respectively and for toxicity (disseminated intravascular coagulation) in another patient after 2 months. These patients underwent HSCT. One patient died of graft failure 3 months after HSCT. Overall, eleven patients (91%) were alive 13.4±5.6 months after eltrombopag onset. Disseminated intravascular coagulation occurred in a 2 years patient who received a large ELT dosage (12.5 mg/Kg). No other grade III-IV toxicity was reported.
Discussion/Conclusion: This is the first report of ELT for childhood severe AAA after IST. Eltrombopag was overall well tolerated. We observed a high rate of sustained trilineage response as reported in adult patients and only 1 case of clonal evolution which is a known complication in non-responsive AAA. Onset of the trilinear hematologic responses is progressive and achieved 3-6 months after ELT initiation. A longer follow-up of this cohort is mandatory to assess response durability and clonal evolution final risk. Nevertheless, we propose that ELT should be considered for severe AAA non-responsive to IST after 3 months of treatment especially if only an alternative donor is available.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal